TURN YOUR PASSIONS INTO ACTION.
BECAUSE EVERY KID DESERVES TO GROW UP.
CREATE YOUR OWN FUNDRAISER
Do it your way! The sky’s the limit! You can turn your birthday, anniversary, or graduation into a celebration that also helps kids! Maybe you are athletic and run or bike or maybe you prefer to host a bake sale. Turn your passions into action by creating a fundraiser to support research for the most rare childhood cancers.

ANNUAL LACE UP FOR KIDS
Our most popular annual fundraiser! September is recognized as National Childhood Cancer Awareness Month so get ready to swap out your regular shoelaces for gold ones and shine light on the realities of childhood cancer while raising funds for the development of new treatments.

BECOME A MONTHLY DONOR
Monthly gifts, no matter the size, help sustain the work of SKC to advance new treatments and advocate for increased access all year long. Making smaller monthly gifts can help donors spread an impactful contribution over several months.

DONOR ADVISED FUNDS
A donor-advised fund (DAF) is a simple, tax-smart investment solution for charitable giving. Individuals contribute cash, stocks, or other assets into their DAF and receive an immediate tax deduction. Individuals can recommend donations to charities of their choice over time. DAFs are the fastest growing charitable giving vehicle to support charities like Solving Kids' Cancer.

DONATE A VEHICLE
Donate your car, truck, boat, motorcycle, snowmobile, or jet-ski to support SKC and receive a tax-deduction! This no-cost, no-hassle process begins through our fundraising partner CarEasy. Click the button below to get started!

IRA CHARITABLE DONATIONS
If you are over the age of 71, you may give a gift from your IRA as a tax-free distribution to a qualified charity. This can count towards your required minimum distribution without being considered taxable income for you. The deduction then lowers your adjusted gross income. Contact your financial advisor to learn more.
OUR IMPACT
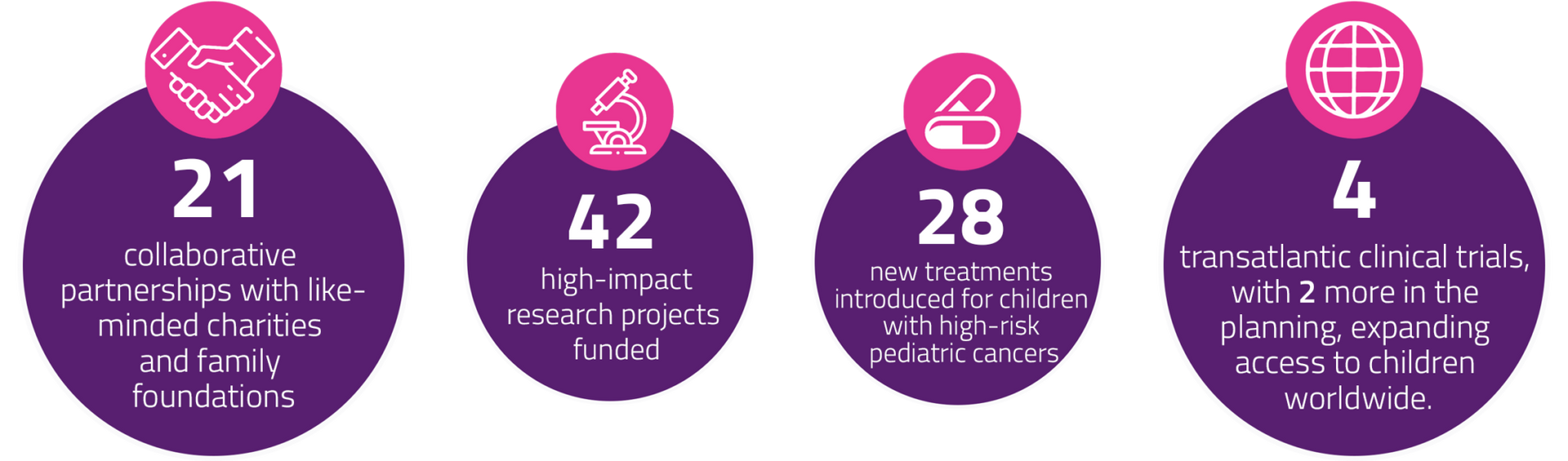
Our donor-driven work is present in more than 250 pediatric cancer centers across 24 countries. By turning your passions into action, you provide hope to families around the world.
BECOME A NONPROFIT PARTNER
Solving Kids’ Cancer has partnered with a number of like-minded nonprofits to collaboratively fund new, innovative treatment options for children battling the most fatal childhood cancers. Together, we can do much more!
Email info@solvingkidscancer.org to learn more about our unique approach to finding, funding, and managing clinical trials.
BECOME A CORPORATE PARTNER
Solving Kids’ Cancer welcomes the opportunity to align with companies who support our mission to cure childhood cancers. Corporate partners help underwrite overhead costs through direct contributions and in-kind services, keeping our focus on developing cures.
Find out how your company can support our continued programs by emailing. info@solvingkidscancer.org.
CONNECT WITH US
HAVE QUESTIONS ABOUT CREATING A FUNDRAISER?
Contact us at info@solvingkidscancer.org.








































































