The Latest in CAR-T Cell Therapy for Pediatric Cancer
Although significant strides have been made in the treatment of children with cancer over the years, survival for some cancers, such as solid tumors, and rare, aggressive cancers, have seen little improvement. Those who do survive pediatric cancers, are often faced with long-term health complications from the current standard of care treatments of chemotherapy, surgery and radiation therapy.1 For children who relapse, survival rate for some cancers is unacceptably low – near 0% – with few second-line treatments available.
But, a promising new cancer treatment that harnesses the power of the immune system may be a game changer.
Chimeric antigen receptor (CAR) T cell therapy is a way of taking the body’s own immune cells, which fight infection, and reprogramming them in a lab to find and kill cancer cells.
Today, CAR-T cell therapy is approved and being used in pediatric oncology for children as well as adolescents and young adults (AYA) with blood cancers, such as lymphoma and certain types of leukemia, often resulting in full remission.2 Researchers are currently working on extending this therapy to improve outcomes for many high-risk pediatric solid tumors.
How Does CAR-T Cell Therapy Work?
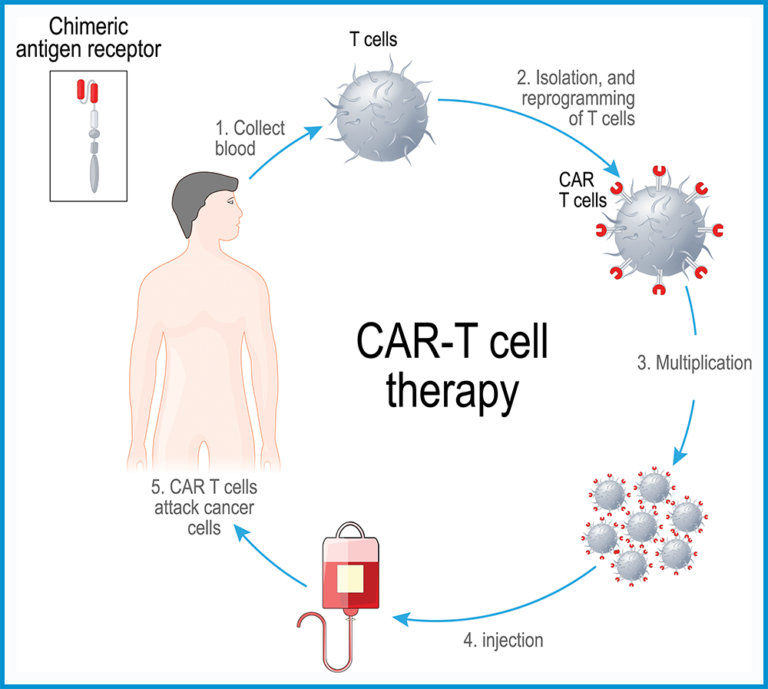
CAR-T cell therapy is a type of immunotherapy using cutting-edge cell engineering to enable the body’s own immune system to heal itself.
The process begins with removing types of white blood cells, called T cells, from the patient’s blood. T cells kill diseased cells by using the protein receptors on their surface to latch onto antigens, which are protein fragments found on the surface of all cells. When a T cell latches onto an abnormal cell, it turns “on” and destroys that cell while minimizing damage to surrounding healthy cells.
But, cancerous cells often look like normal cells. To combat their deception, scientists use CAR-T cell therapy, to attack specific cancer cells by inserting instructions in the patient’s T cells to recognize and latch onto the diseased cell.
Once reprogrammed, the T cells have special receptors called chimeric antigen receptors (CARs) on their surface. Then, these newly engineered CAR-T cells are grown to multiply in a lab. Afterwards, millions of them are returned into the patient’s bloodstream, where the new receptors allow CAR-T cells to seek out and latch on to a specific antigen on the patient’s tumor cells and destroy them.3
Recent Breakthroughs in CAR-T Cell Treatment
Over ten years ago, 6-year-old Emily Whitehead became the first pediatric patient to receive CAR-T cell therapy.5 The treatment was an unprecedented success: the CAR-T cells completely eradicated her acute lymphoblastic leukemia and put her into full remission.
Five years after Emily was treated, the U.S. Food and Drug Administration (FDA) approved the CAR-T cell therapy product used to treat Emily, ushering in a new era in the treatment of cancer. Since then, a total of six CAR-T cell therapies were also approved.
With every patient treated using this novel therapy for cancer, along with continued innovation in CAR-T cell design, exciting new developments in CAR-T cell treatments are leading to more hope for pediatric cancer patients and their families.
Here are the latest breakthroughs showing how CAR-T cells are paving the way to become the next great advancement in cancer treatment:
Continuing Impressive Results from New Cancer Treatment for Blood Cancers
CAR-T cell therapy has resulted in continuing remarkable success for some pediatric blood cancers, such as lymphoma, certain types of leukemia, and most recently, multiple myeloma (MM), the second most common type of blood cancer in the United States. CAR-T cell therapy has shown extraordinary responses with refractory MM. Continuing research is underway to further improve patient outcomes.6
Earlier CAR-T Cell Therapy Produces More Effective Results
Recent, large clinical trials have shown that after initial chemotherapy, CAR-T cell therapy may be more effective than current conventional treatments. With ongoing trials proving similar positive outcomes, researchers have noted that these dramatic results could signal a change in the future with CAR-T cells being used earlier in the course of the disease, which means it could become a frontline treatment for many more types of cancers soon.7
CAR-T Cell Treatment Results in Solid Tumors Are Improving
Solid tumors like diffuse intrinsic pontine glioma (DIPG) tumors are extremely difficult to treat due to its aggressive nature and location in the brain. While CAR-T cell treatment results in solid tumors are limited, progress is improving and heading in the right direction.
The latest developments include the first patient infused directly with CAR-T cells into the brain to treat DIPG, which resulted in both proving the treatment was tolerable and that it inhibited growth of the cancer cells for a period of time.8
Also, a recent collaboration of using GD-2 (a sugar molecule found on the surface of DIPG tumors) with CAR-T cell therapies in neuroblastoma and central nervous system gliomas have shown promising results in several clinical trials with dramatic reduction in size of DIPG tumors and improvement in symptoms.
CAR-T Cell Treatment of Relapsed or Refractory Cancers Is Underway
In 2020, the FDA approved the first CAR-T cell treatment for relapsed and refractory cancer. Some cases of these groups of clinical trials resulted in CAR-T cell therapy being so effective that they didn’t require further types of treatments.9 CAR-T cells as first- or second-line of treatment could significantly transform the treatment picture for relapsed or refractory cancers that conventional therapies initially fail to cure.10
Solving Kids’ Cancer’s Role in Advancing Research in Pediatric Cancer Treatment
Over a decade since receiving CAR-T cell therapy, Emily Whitehead is now a bright 17-year-old applying for college. Her 10-year survival shows the incredible progress of this new cancer treatment and the long-term hope it offers. Although there is still much work to be done, CAR-T cells have been a lifesaving treatment to many, like Emily, who only a few years ago would have succumbed to their disease.
At Solving Kids’ Cancer, we believe the cures are in the science. That’s why we’re committed to funding breakthrough research and clinical trials to find cures for children with the deadliest childhood cancers.
Over the past decade, we have funded three DIPG cancer-specific clinical trials and eight additional trials that included the study of DIPG and other types of pediatric brain tumors. Additionally, one of our more recent landmark pediatric research projects involves testing a new CAR-T cell therapy specifically targeting ETMR and medulloblastoma cancer cells.
Help us raise awareness and funding to give more kids a second chance at life — because every kid deserves to grow up.
Sources
1 Childhood Cancer Fact Library https://cac2.org/interest-groups/awareness/childhood-cancer-fact-library/
2 Study Shows CAR T-Cell Therapy Is Effective at Putting Childhood Leukemia Into Remission
https://www.cancer.net/blog/2022-12/study-shows-car-t-cell-therapy-effective-putting-childhood-leukemia-remission#:~:text=Leukemia%20Into%20Remission-,Study%20Shows%20CAR%20T%2DCell%20Therapy%20Is%20Effective,Putting%20Childhood%20Leukemia%20Into%20Remission&text=Results%20from%20a%20recent%20study,children%20with%20high%2Drisk%20disease.
3 CAR T-cell Therapy and Its Side Effects https://www.cancer.org/treatment/treatments-and-side-effects/treatment-types/immunotherapy/car-t-cell1.html
4 First-Ever CAR T-cell Therapy Approved in U.S. https://aacrjournals.org/cancerdiscovery/article/7/10/OF1/6073/First-Ever-CAR-T-cell-Therapy-Approved-in-U-S
5 Emily Whitehead, First Pediatric Patient to Receive CAR T-Cell Therapy, Celebrates Cure 10 Years Later https://www.chop.edu/news/emily-whitehead-first-pediatric-patient-receive-car-t-cell-therapy-celebrates-cure-10-years#:~:text=Search-,Emily%20Whitehead%2C%20First%20Pediatric%20Patient%20to%20Receive%20CAR%20T%2DCell,Celebrates%20Cure%2010%20Years%20Later
6 CAR T-Cell Therapy for Patients with Multiple Myeloma: Current Evidence and Challenges https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9439649/
7 Should CAR T Cells Be Used Earlier in People with Non-Hodgkin Lymphoma? https://www.cancer.gov/news-events/cancer-currents-blog/2022/nhl-car-t-cells-belinda-transform-zuma7
8 Could CAR-T-cell therapy offer hope to children with cancer? https://www.nature.com/articles/d41586-022-04344-6
9 CAR T-cell therapy boosts cure rates for children with aggressive blood cancer https://connect.uclahealth.org/2022/11/03/car-t-cell-therapy-boosts-cure-rates-for-children-with-aggressive-blood-cancer/
10 Paediatric Strategy Forum for medicinal product development of chimeric antigen receptor T-cells in children and adolescents with cancer: ACCELERATE in collaboration with the European Medicines Agency with participation of the Food and Drug Administration
https://pubmed.ncbi.nlm.nih.gov/34840026/



















































































