THE LATEST
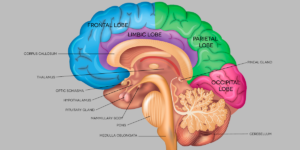
Rare Brain Tumors in Kids
Brain and spinal cord tumors, also known as central nervous system (CNS) tumors, are the most common type of solid tumors affecting children. Brain tumors make up about 15% of pediatric cancers, and are the second most commonly diagnosed cancer in children and now, the deadliest type of childhood cancer, ahead of leukemia.
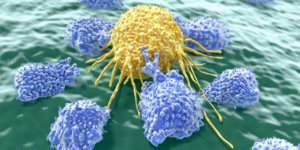
Clinical Trial Harnesses Power of Natural Killer Cells to Treat Neuroblastoma
Solving Kids’ Cancer, The Catherine Elizabeth Blair Memorial Foundation, and Wade’s Army awarded a $136,000 grant to support the novel immunotherapy clinical trial for childhood cancer. Researchers will use a humanized monoclonal antibody linked to IL2, known as hu14.18-IL2, which specifically targets neuroblastoma tumor cells and binds to them, while the IL2 activates NK cells…
A Welcome Gift for the Pediatric Brain Tumor Community
What began as a proposal for a four-center US-based clinical trial of promising combination therapy for children battling brain tumors, has expanded into a large-scale clinical trial at 58 centers in 13 countries, that brings new hope to children across the globe.
Targeted Therapy for Children with ALK-Driven Neuroblastoma
Lorlatinib, an investigational drug candidate currently in late-stage clinical development for the treatment of lung cancer may also prove effective for the treatment of the pediatric cancer neuroblastoma. Solving Kids’ Cancer is leading an international effort of like-minded nonprofits to provide nearly $400,000 of collaborative funding for an innovative clinical trial to bring a potentially life-saving therapy to kids.
Advocacy in Action
Solving Kids’ Cancer was the first charity to ever go against the UK healthcare system without a pharmaceutical company involved. As a result, the National Institute for Health and Care Excellence (NICE) recently announced that it has upheld SKC’s appeal against the Institute’s decision not to recommend dinutuximab for treating high-risk neuroblastoma.
Precision Medicine Advances for Children With Neuroblastoma
Solving Kids’ Cancer (SKC) together with Wade’s Army announce their joint financial support of this Phase 1 clinical trial to test the safety and efficacy of various investigational drugs that will leverage precision medicine for children with neuroblastoma. This Next Generation Personalized Neuroblastoma Therapy – also known as NEPENTHE (Greek for “medicine for sorrow”) – is the first precision medicine trial for children that will robustly analyze the genomics of their cancer and use combinations of investigational drugs to target specific mutations in their tumors.
Children with High Grade Brain Tumors will be able to Receive Cutting-edge Oncolytic Virus Therapy
The Andrew McDonough B+ Foundation (The B+ Foundation) and Solving Kids’ Cancer (SKC) presented a check for $208,500 to Dr. Matthias Gromeier and Dr. Eric Thompson at the Preston Robert Tisch Brain Tumor Center at Duke, to co-fund an exciting new immunotherapy trial for children with deadly brain tumors.
The Launch of an International Immunotherapy Trial for Children with Brain Tumors
A Kids’ Brain Tumor Cure Foundation, Solving Kids’ Cancer, and the Ty Louis Campbell (TLC) Foundation announce their joint financial support of a Phase 1 clinical trial to test the safety and efficacy of combination checkpoint inhibitors in the treatment of children with brain tumors.
SKC Formally Appeals NICE Decision
The National Institute for Health and Care Excellence (NICE) heard an appeal led by Solving Kids’ Cancer (SKC) against its recent decision to deny children in the UK treatment that is proven to significantly increase the chance of survival for children diagnosed with an aggressive childhood cancer, neuroblastoma.